
QIBEBT Developed Novel Electrochemical Sensors Based on Ordered Mesoporous Carbons
Apr 24, 2014 Email"> PrintText Size
Functional nanostructured materials are extensively studied aiming at improving the sensitivity and selectivity of the chemical/biosensors. The non-silicon based ordered mesoporous carbons (OMCs) are a kind of metastable carbon crystals with large surface area, well-defined pore size, and flexible framework. Recently, an electrochemical sensing system based on OMCs was developed by researchers from Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences (CAS-QIBEBT) and employed to selectively and sensitively detect nitrophenol isomers and nitrophenyl organophosphates in seawater and wastewater. The related results have been published in Electrochimica Acta and Analytica Chimica Acta.
Using hard-template method, LANG Qiaolin and ZHANG Tingting from Biosensing Group of Bioenergy Center synthesized the OMCs, which structure was shown in Figure 1. They found that the OMCs modified glassy carbon electrode (OMCs/GCE) exhibited enhanced electrocatalytic activity towards the reduction of nitrophenol isomers (p-NP, m-NP and o-NP). Interestingly, at OMCs/GCE, the three nitrophenol isomers could be identified and separated successfully and the simultaneous determination was realized by detecting the reduction peaks of their intermediate products with differential pulse voltammetry (DPV) (Figure 2). And common inorganic ions and organic compounds had no influence on the signals of nitrophenol isomers. This proposed electrochemical sensor was feasible for the simultaneous detection of nitrophenol isomers in seawater and wastewater (Electrochimica Acta 2013, 106, 127-134).
Further, the ultrasensitive and selective electrochemical sensor for p-nitrophenyl organophosphates based on OMCs was also developed by this team. By using DPV technique, the OMCs/GCE can be used to detect p-nitrophenyl organophosphates including paraoxon, parathion and methyl parathion, with the lower limit of detection up to nanomolar level (Figure 3). And it was proved that the oxygen reduction on the OMCs/GCE had no influence on the detection of p-nitrophenyl OPs, which is a great advantage over other similar methods reported so far. The prepared sensor is expected to have wide application in on-site p-nitrophenyl OPs detection in seawater and wastewater (Analytica Chimica Acta 2014, 822, 23–29).
In addition, this research team selected p-nitrophenol and p-methylphenol as models of electron-withdrawing and electron-donating groups, respectively to study the electrochemical behavior of phenols and explored the electrooxidation mechanism of phenols at OMCs/GCE. Based on the relationship between pKa and Epa, a novel and simple equation was proposed for precise calculation of the pKa of p-substituted and o-substituted phenols (Electrochimica Acta 2014, 115, 283–289).
This work was financially supported by National Natural Science Foundation of China, which was hosted by Prof. LIU Aihua, leader of the Biosensing Group at the QIBEBT. And the group of Associate Prof. LI Tie from Ocean University of China and the group of Prof. WEI Mingdeng from Fuzhou University, China, also participated in this work.
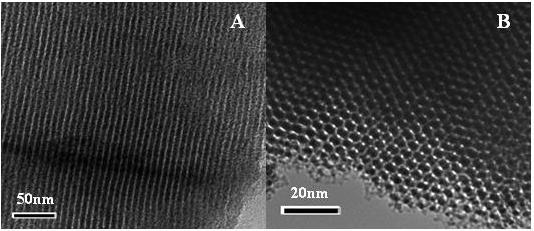
Figure 1. (A) TEM image showing mesopore channel structure. (B) TEM image of the mesoporous carbon along the [001] direction. (Image by Prof. LIU's group) 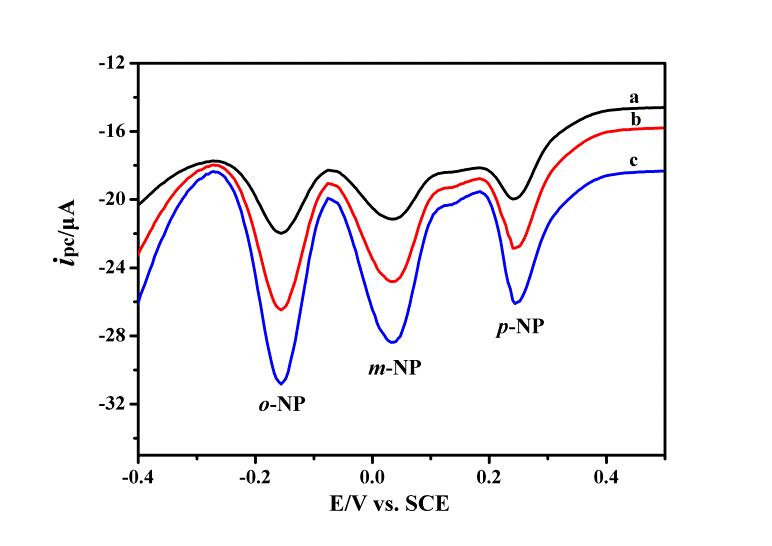
Figure 2. DPV responses of three nitrophenol isomers at OMCs/GCE in 0.1 M PBS (pH 4.8). (a) 10 μM; (b) 20μM; and (c) 30 μM of o-nitrophenol (o-NP), m-nitrophenol (m-NP) and p-nitrophenol (p-NP). DPV conditions: equilibration time, 2 s; potential amplitude, 50 mV; pulse period: 0.5 s; step height, 4 mV; frequency, 60Hz. (Image by Prof. LIU's group)
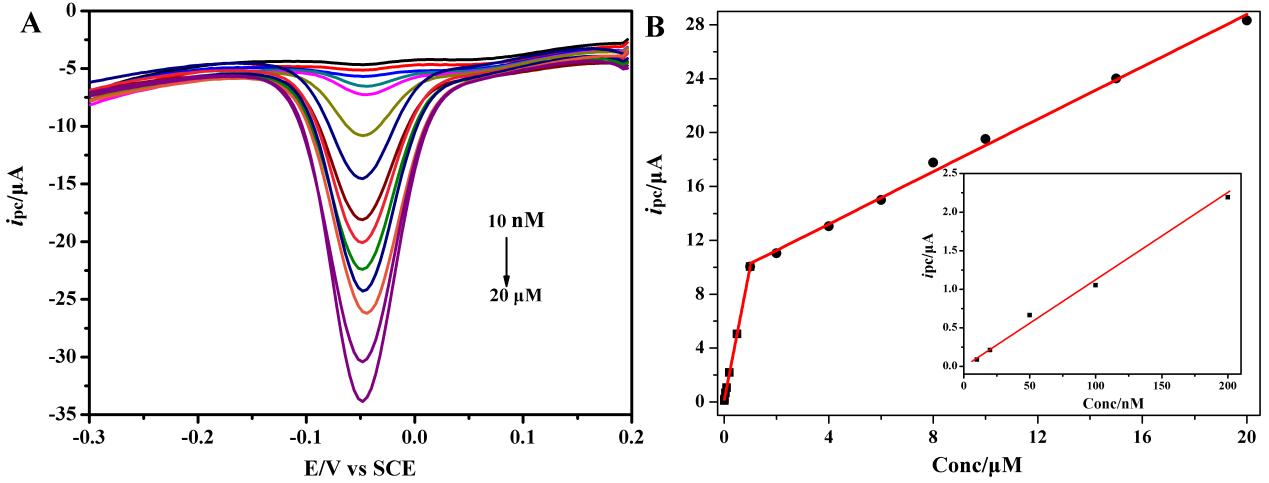
Figure 3. (A) DPVs of paraoxon varying concentration at OMCs/GCE in 0.1M PBS (pH 6.0). DPV conditions: equilibration time, 2 s; potential amplitude, 50 mV; pulse period: 0.5 s; step height, 4 mV; frequency, 60Hz. (B) The calibration curve for paraoxon. Inset, the enlarged portion of the current as a function of paraoxon concentration within 10-200 nM. (Image by Prof. LIU's group)
Link of the publications:
1. Electrochimica Acta 2013, 106, 127-134.
2. Analytica Chimica Acta 2014, 822, 23–29.
3. Electrochimica Acta 2014, 115, 283–289.
Contact:
Professor LIU Aihua
E-mail: liuah (AT) qibebt.ac.cn
Functional nanostructured materials are extensively studied aiming at improving the sensitivity and selectivity of the chemical/biosensors. The non-silicon based ordered mesoporous carbons (OMCs) are a kind of metastable carbon crystals with large surface area, well-defined pore size, and flexible framework. Recently, an electrochemical sensing system based on OMCs was developed by researchers from Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences (CAS-QIBEBT) and employed to selectively and sensitively detect nitrophenol isomers and nitrophenyl organophosphates in seawater and wastewater. The related results have been published in Electrochimica Acta and Analytica Chimica Acta.
Using hard-template method, LANG Qiaolin and ZHANG Tingting from Biosensing Group of Bioenergy Center synthesized the OMCs, which structure was shown in Figure 1. They found that the OMCs modified glassy carbon electrode (OMCs/GCE) exhibited enhanced electrocatalytic activity towards the reduction of nitrophenol isomers (p-NP, m-NP and o-NP). Interestingly, at OMCs/GCE, the three nitrophenol isomers could be identified and separated successfully and the simultaneous determination was realized by detecting the reduction peaks of their intermediate products with differential pulse voltammetry (DPV) (Figure 2). And common inorganic ions and organic compounds had no influence on the signals of nitrophenol isomers. This proposed electrochemical sensor was feasible for the simultaneous detection of nitrophenol isomers in seawater and wastewater (Electrochimica Acta 2013, 106, 127-134).
Further, the ultrasensitive and selective electrochemical sensor for p-nitrophenyl organophosphates based on OMCs was also developed by this team. By using DPV technique, the OMCs/GCE can be used to detect p-nitrophenyl organophosphates including paraoxon, parathion and methyl parathion, with the lower limit of detection up to nanomolar level (Figure 3). And it was proved that the oxygen reduction on the OMCs/GCE had no influence on the detection of p-nitrophenyl OPs, which is a great advantage over other similar methods reported so far. The prepared sensor is expected to have wide application in on-site p-nitrophenyl OPs detection in seawater and wastewater (Analytica Chimica Acta 2014, 822, 23–29).
In addition, this research team selected p-nitrophenol and p-methylphenol as models of electron-withdrawing and electron-donating groups, respectively to study the electrochemical behavior of phenols and explored the electrooxidation mechanism of phenols at OMCs/GCE. Based on the relationship between pKa and Epa, a novel and simple equation was proposed for precise calculation of the pKa of p-substituted and o-substituted phenols (Electrochimica Acta 2014, 115, 283–289).
This work was financially supported by National Natural Science Foundation of China, which was hosted by Prof. LIU Aihua, leader of the Biosensing Group at the QIBEBT. And the group of Associate Prof. LI Tie from Ocean University of China and the group of Prof. WEI Mingdeng from Fuzhou University, China, also participated in this work.
|
|
| Figure 1. (A) TEM image showing mesopore channel structure. (B) TEM image of the mesoporous carbon along the [001] direction. (Image by Prof. LIU's group) |
|
|
| Figure 2. DPV responses of three nitrophenol isomers at OMCs/GCE in 0.1 M PBS (pH 4.8). (a) 10 μM; (b) 20μM; and (c) 30 μM of o-nitrophenol (o-NP), m-nitrophenol (m-NP) and p-nitrophenol (p-NP). DPV conditions: equilibration time, 2 s; potential amplitude, 50 mV; pulse period: 0.5 s; step height, 4 mV; frequency, 60Hz. (Image by Prof. LIU's group) |
 |
| Figure 3. (A) DPVs of paraoxon varying concentration at OMCs/GCE in 0.1M PBS (pH 6.0). DPV conditions: equilibration time, 2 s; potential amplitude, 50 mV; pulse period: 0.5 s; step height, 4 mV; frequency, 60Hz. (B) The calibration curve for paraoxon. Inset, the enlarged portion of the current as a function of paraoxon concentration within 10-200 nM. (Image by Prof. LIU's group) |
Link of the publications:
1. Electrochimica Acta 2013, 106, 127-134.
2. Analytica Chimica Acta 2014, 822, 23–29.
3. Electrochimica Acta 2014, 115, 283–289.
Contact:
Professor LIU Aihua
E-mail: liuah (AT) qibebt.ac.cn
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

