
Researchers Report High-nuclearity Pure Lanthanide Clusters
Apr 09, 2014 Email"> PrintText Size
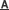
The polynuclear pure lanthanide compounds have important applications in the organic electroluminescence, fluorescence imaging, molecule-based magnetic materials and so on. However, the synthesis of high-nuclearity pure lanthanide clusters, especially over thirty nuclears, continues to be a challenge. The most important reason may be that lanthanide ions have variable and high coordination numbers as well as poor directionality.
The research group headed by Prof. HONG Maochun at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, have developed a strategy for the syntheses of the high-nuclearity lanthanide clusters in the presence of different anion templates by the slow hydrolysis of lanthanide ions under the hydrothermal condition.
A polymeric tube-like Er48 complex has been synthesized and structurally characterized, which can be viewed as the aggregation of two types of cluster units, i.e. one ring-like Er12 unit and two identical wheel-like Er18 units. A particularly salient feature of this Er48 cluster is that two kinds of anions (Cl- and NO3-) template the formation of the Er18 wheels and the Er12 ring through hydrogen bonding respectively. Results of this study have been published as a communication in Chem. Commun. (Chem. Commun., 2014, DOI:10.1039/c3cc46779a).
Using this method, two 36-nuclearity lanthanide clusters have been synthesized and reported before. Gd36 possesses a large MCE of 39.66 J Kg-1 K-1 and 2 exhibits slow relaxation of the magnetization (Chemical Science, 2013, 4, 3104-3109).
Considering that the above double-anion templates not only effectively increase the nuclearity of the clusters but also donate much more novelty to the final structures, these results are thought to be able to throw light on the research of double- or multi-anion templating effects on the syntheses of high-nuclearity lanthanide clusters.
The sketch map of the Cl- and NO3- anions templating the formation of the nano-size Er48 tube through hydrogen bonding. (Image by Prof. HONG’s group)
The polynuclear pure lanthanide compounds have important applications in the organic electroluminescence, fluorescence imaging, molecule-based magnetic materials and so on. However, the synthesis of high-nuclearity pure lanthanide clusters, especially over thirty nuclears, continues to be a challenge. The most important reason may be that lanthanide ions have variable and high coordination numbers as well as poor directionality.
The research group headed by Prof. HONG Maochun at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, have developed a strategy for the syntheses of the high-nuclearity lanthanide clusters in the presence of different anion templates by the slow hydrolysis of lanthanide ions under the hydrothermal condition.
A polymeric tube-like Er48 complex has been synthesized and structurally characterized, which can be viewed as the aggregation of two types of cluster units, i.e. one ring-like Er12 unit and two identical wheel-like Er18 units. A particularly salient feature of this Er48 cluster is that two kinds of anions (Cl- and NO3-) template the formation of the Er18 wheels and the Er12 ring through hydrogen bonding respectively. Results of this study have been published as a communication in Chem. Commun. (Chem. Commun., 2014, DOI:10.1039/c3cc46779a).
Using this method, two 36-nuclearity lanthanide clusters have been synthesized and reported before. Gd36 possesses a large MCE of 39.66 J Kg-1 K-1 and 2 exhibits slow relaxation of the magnetization (Chemical Science, 2013, 4, 3104-3109).
Considering that the above double-anion templates not only effectively increase the nuclearity of the clusters but also donate much more novelty to the final structures, these results are thought to be able to throw light on the research of double- or multi-anion templating effects on the syntheses of high-nuclearity lanthanide clusters.

The sketch map of the Cl- and NO3- anions templating the formation of the nano-size Er48 tube through hydrogen bonding. (Image by Prof. HONG’s group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

