

A research team led by Prof. XUE Tian from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences revealed the neural mechanism of photoreception suppressing thermogenesis in brown adipose tissue (BAT), thereby reducing glucose tolerance (GT) in mice and humans. This work was published in Cell.
Previous studies indicated that light is a substantial factor for metabolic disorders. However, whether the metabolic modulation by light is direct or through disruptions in circadian rhythms remains unknown. Moreover, the specific processes and neural circuit involved in the light modulation of glucose metabolism are yet to be determined.
To shed light on these problems, Prof. XUE’s team first performed glucose tolerance tests on both mice and humans, and found that their GT decreased significantly under light. Besides cones and rods that mediate visual perception, light can directly activate intrinsically photosensitive retinal ganglion cells (ipRGCs), which innervate many brain regions and thus regulate functions like circadian rhythm. To determine which type of photoreceptor mediates light-induced glucose intolerance, the team disabled different types of photoreceptors one by one and found that it is mediated by ipRGCs only.
To determine whether glucose intolerance is caused by light-induced circadian rhythm disruptions or direct light modulation through neural circuit, the team probed the central circadian pacemaker and hypothalamus supraoptic nucleus (SON) separately with an excitotoxic lesion to observe their contribution to the decrease of GT. The results showed that light-induced glucose intolerance is mediated by ipRGC-SON pathway, independent of circadian phase. The team also discovered the ipRGC-SON-PVN-NTS-RPa pathway that mediates light-induced glucose intolerance using neural circuit tracing techniques.
Besides, the team explored the light-induced changes of hormones and nutrients involved in glucose metabolism, including insulin, glucagon and glucagon like peptide-1, and found that they remained the same. Thus, light does not interfere with glycogen synthesis and catabolism. The results also showed no changes in the levels of lactic acid, nonesterified fatty acids and other amino acids in the blood, eliminating the possibility of light suppressing the conversion of blood glucose to fat and amino acids.
Therefore, the team focused on the respiration of BAT, which consumes glucose and fat to produce heat to maintain body temperature homeostasis. Using techniques including blocking the sympathetic projections to BAT, the team confirmed that light-induced glucose intolerance is caused by the suppression of thermogenesis in BAT by light, which reduces glucose consumption, and found that subjects in thermoneutral environment show no signs of glucose intolerance, indicating that light-induced glucose intolerance may also be mediated by BAT activity.
This study revealed the specific neural pathway of light modulation on glucose metabolism, which can serve as potential therapeutic targets for metabolic diseases in future research.
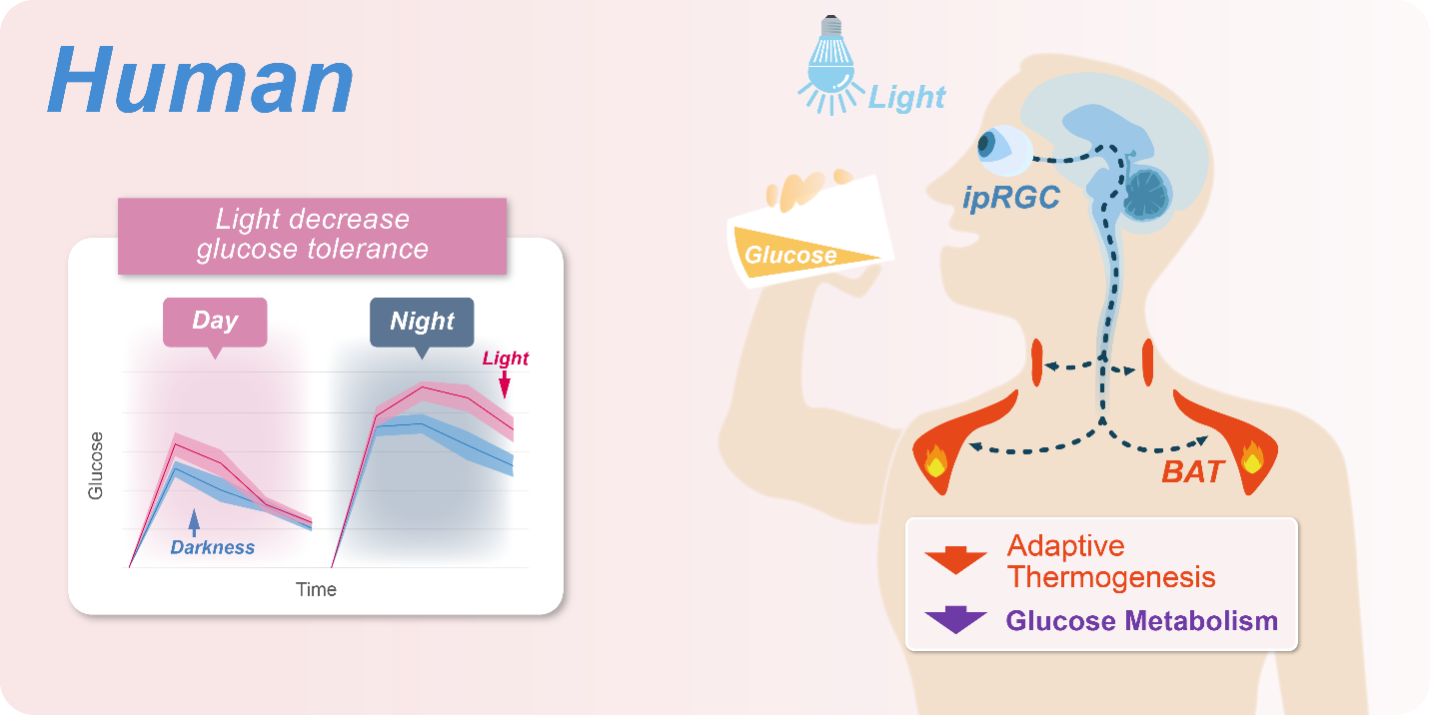
GT in human can be reduced by the same neural pathway which suppresses the BAT thermogenesis. (Image by Prof. XUE's team)