A healthy baby boy was born in Nanjing Drum Tower Hospital on Jan. 12, 2018. The birth of the baby is the fruit of the world's first clinical trial to treat premature ovarian failure (POF) with umbilical cord mesenchymal stem cells combined with a collagen scaffold. This research was jointly conducted by the Reproductive Medicine Research Team of the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (CAS) and Nanjing Drum Tower Hospital. It represents a significant breakthrough in the application of China’s stem cell and regenerative medicine technology to the life sciences and health field.
The research team at the Guangzhou Institutes of Biomedicine and Health of CAS developed a simple, efficient and standardized method for stem cell preparation. The method offers a new scientific perspective and solution for inducing pluripotent stem cells and optimizing their preparation. The "Automated Stem Cell Induction and Culturing System" is the first automated stem cell induction culturing equipment with its own proprietary intellectual property rights, and the first automated preparation equipment for inducing pluripotent stem cells for therapeutic purposes.
Researchers from the Shanghai Institute of Nutrition and Health of CAS characterized the entire process of hematopoietic stem cell (HSC) homing in vivo and discovered a novel subpopulation of macrophages, called "Usher cells," which can recognize and guide HSC into a vascular niche. This work was published as a cover story in Nature on Dec 6, 2018, providing new insights for improving the efficiency of HSC transplantation.
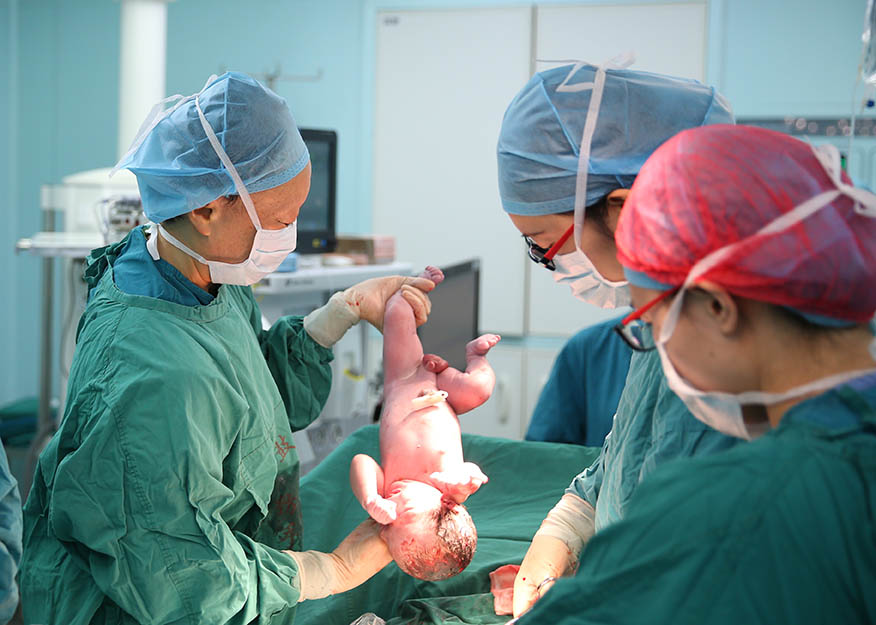
World's first clinical trial to treat premature ovarian failure (POF)

Automated Stem Cell Induction and Culturing System
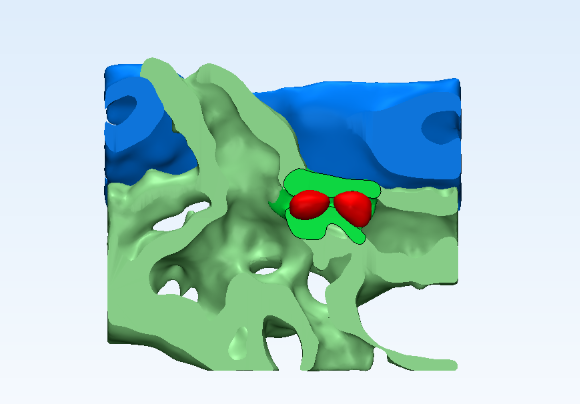
Hematopoietic stem cell (HSC) homing in vivo
Related Articles:
