Sodium oligomannate (GV-971), an innovative, orally administered drug for treating Alzheimer's disease, completed its phase III clinical trial in July 2018. GV-971 is the fruit of a 21-year research effort by a team led by Prof. GENG Meiyu, principle investigator at the Shanghai Institute of Materia Medica.
The phase III trial of GV-971 met its primary endpoint to improve cognition impairment, with significant statistical differences and clinical benefits. GV-971 treatment was safe and well tolerated in patients, with no statistically significant difference in adverse effects compared with the placebo group, supporting its safety for long-term use.
GV-971 is the only drug anywhere in the world to meet the endpoint of phase III clinical trials for treating Alzheimer's disease during the past 16 years. The drug was jointly developed by the Ocean University of China, the Shanghai Institute of Materia Medica and Green Valley Pharmaceutical Co., Ltd.
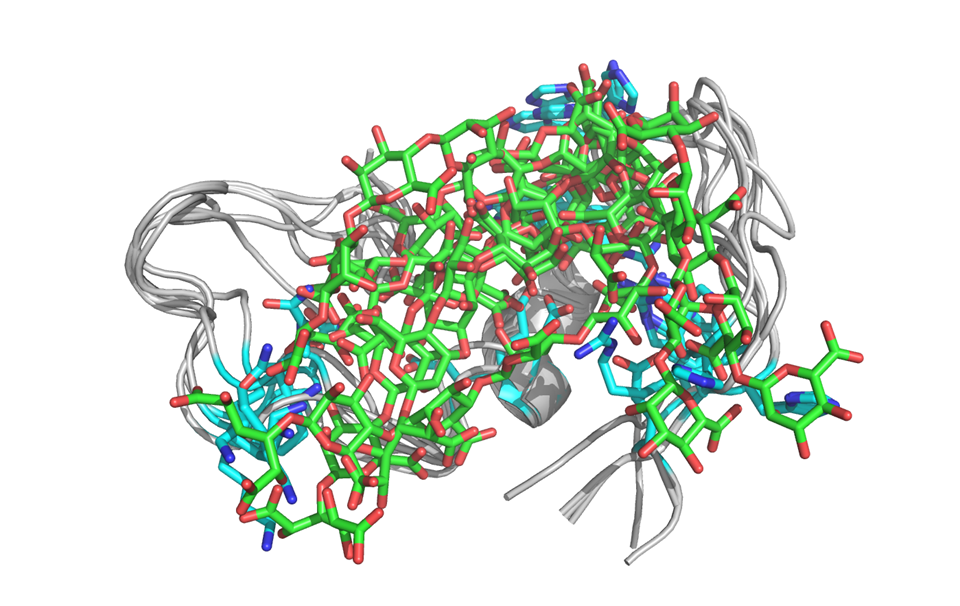
Sodium oligomannate (GV-971)
Related Articles:
China's New Alzheimer's Drug Completes Phase 3 Clinical Trial