
One of the major constituents of plant biomass, D-xylose, has been considered as an attractive carbon source for bio-based fuel and chemical production through fermentation. But for years people did not know how the environmental D-xylose signal is sensed by the bacteria and metabolize D-xylose in detail.
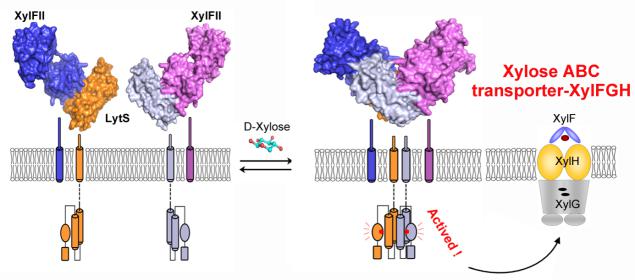
In 2015, a membrane two-component complex was identified in Firmicutes bacteria, comprising a membrane-associated sensor protein (XylFII), a transmembrane histidine kinase (LytS) for periplasmic d-xylose sensing, and a cytoplasmic response regulator (YesN) that activates the transcription of the target ABC transporter XylFGH genes to promote the uptake of d-xylose. However, the molecular mechanism underlying signal perception and integration of these processes remains unclear.
In a recent study published in PNAS, a research team led by Profs. ZHANG Peng and JIANG Weihong at Institute of Plant Physiology and Ecology of Chinese Academy of Sciences determined the structures of the XylFII-LytSN (N-terminal periplasmic domain of LytS) complex in its d-xylose free and d-xylose bound forms, which together with biochemical and physiological methods demonstrated the D-xylose sensing mechanism through XylFII-LytS in bacteria.
Researchers found that LytSN contains a four-helix bundle, and XylFII contains two Rossmann fold-like globular domains with a xylose binding cleft between them. In the absence of d-xylose, LytSN and XylFII can form a heterodimer.
Specific binding of d-xylose to the cleft of XylFII will induce a large conformational change that will close the cleft and bring the globular domains closer together. This conformational change can lead to the formation of an active XylFII-LytS heterotetramer, which activates the downstream response regulator and the expression of the d-xylose ABC transporter XylFGH.
They further revealed that mutations at the d-xylose binding site and the heterotetramer interface will diminish heterotetramer formation and impair the d-xylose–sensing function of XylFII-LytS. Based on these data, a working model of XylFII-LytS was proposed that can provides a molecular basis for d-xylose utilization and metabolic modification in bacteria.
Hopefully, this study will contribute to the development of d-xylose utilization and metabolic engineering in bacteria.
This work was funded by the National Natural Science Foundation of China, and Chinese Academy of Sciences.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

