
Chinese researchers realized analysis of metastable nanoparticles and probed the kinetics of phase transition of amorphous Se in different solvents on a platform.
They used in-situ dynamic spectrum system to the newly developed platform on their own for the research.
Dynamic monitoring and analysis of the intrinsic growth process of metastable nanoparticles is the key to understand the formation mechanism of nanocrystals.
Based on the in-situ dynamic spectrum system for analysis of metastable nanoparticles, the phase transition of uncapped selenium (Se) colloids in three typical polar aprotic solvents were dynamically monitored, including dimethyl formamide, acetone and ethyl acetate.
As analyzed, the rate of phase transition was displayed as follows: dimethyl formamide > acetone > ethyl acetate.
These findings demonstrated that polar aprotic solvents dominated the kinetics of phase transition, and gave credible data for understanding the real situation of uncapped NPs in colloidal solution.
This research was supported by National Basic Research Program of China, Instrument Developing Project of the Chinese Academy of Sciences, National Natural Science Foundation of China.
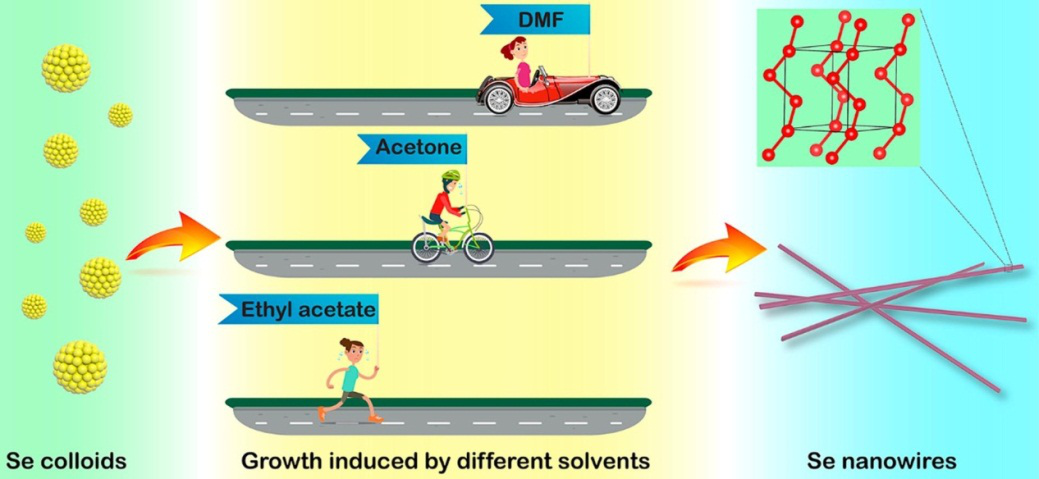
Solvent molecules dominated phase transition from uncapped amorphous Se colloids to trigonal Se nanowires (Image by CHEN Qi)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

