
In the simplest chemical reaction in nature, the H + H2 reaction, a well-known conical intersection exists between the ground and first excited state. Therefore, the H + H2 reaction and its isotopic variants have long been the benchmark system in the study of the geometric phase (GP) effect in chemical reactions.
Previously, efforts were made to observe and understand the GP effect in the H+H2 reaction. However, no convincing experimental evidence of the GP effect in any chemical reaction has been detected until now.
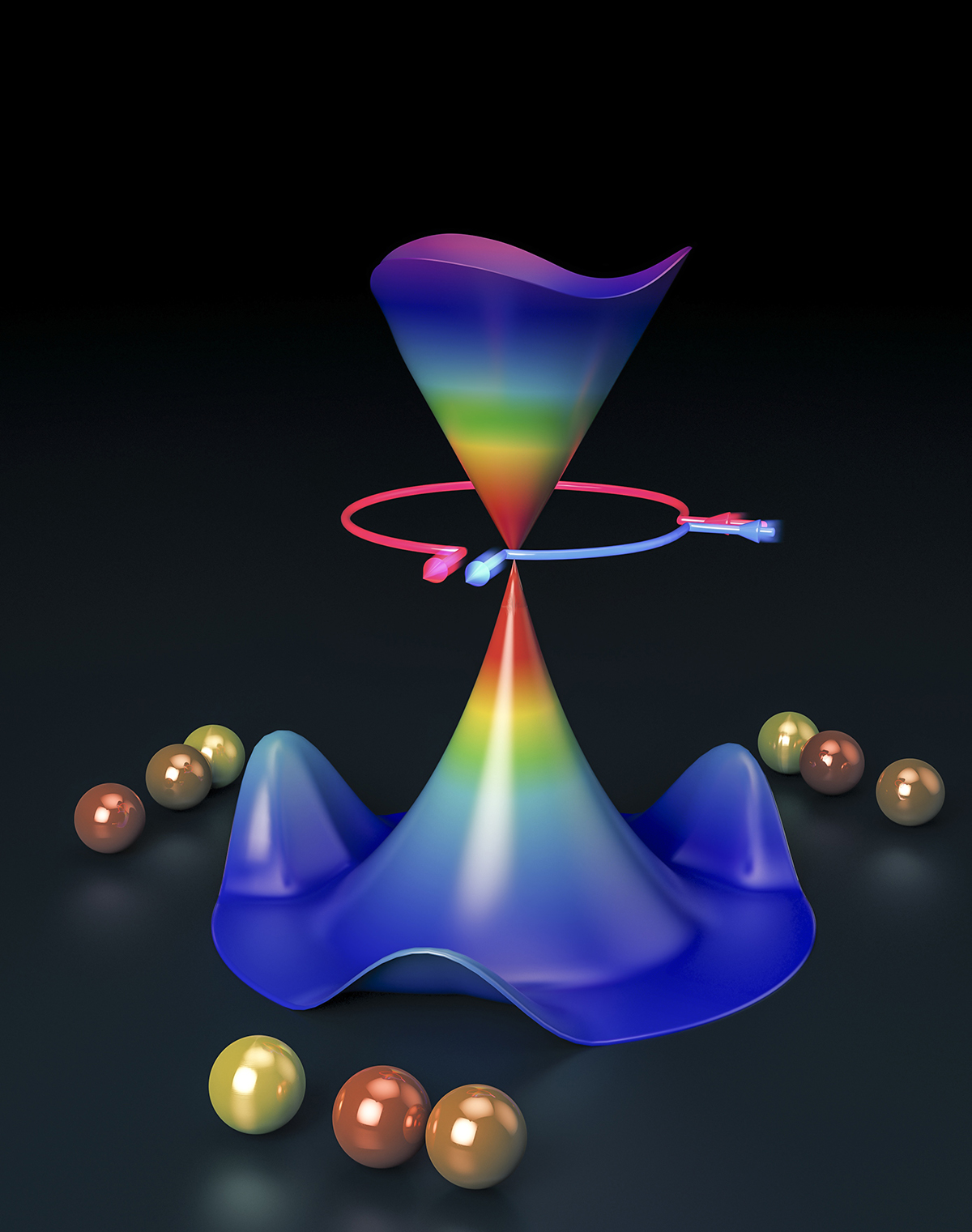
Schematic illustration of the geometric phase effect arising in the H+HD reaction: the different interference of two reaction path. (Image by SUN Zhigang)
Recently, researchers from the University of Science and Technology of China and the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences carried out a combined experimental and theoretical investigation of the H+HD→H2+D reaction.
The experimental team, which was led by Prof. WANG Xingan and Prof. YANG Xueming, performed a crossed molecular beams study using the high-resolution velocity map ion imaging technique. Rapid forward-scattering oscillations of H2 (v', j') products were observed in differential cross sections at a collision energy in the vicinity of the H3 conical intersection.
Prof. SUN Zhigang developed a unique quantum theoretical approach for considering the GP effect in a chemical reaction. Based upon a newly developed accurate potential energy surface by Prof. ZHANG Donghui, the researchers found that the experimentally observed oscillation structures in the forward scatterings could only be reproduced by theoretical calculations including the geometric phase effect.
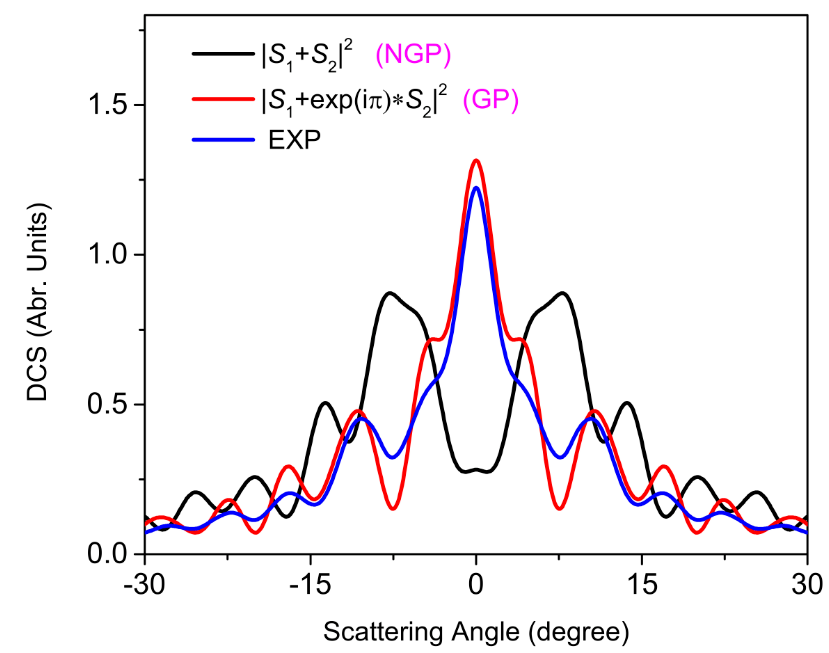
The interference with GP is out of phase from that without the GP. (Image by SUN Zhigang)
Through this study, a new reaction mechanism has also been discovered for this benchmark reaction at high collision. This investigation clearly answered a long- standing question in chemical reaction dynamics, i.e., how the GP effect profoundly influences chemical reactivity. The study certainly has important implications for dynamics studies of molecular systems with conical intersections in general.
This research, entitled "Observation of the geometric phase effect in the H+HD→H2+D reaction," was published in Science.
The Born-Oppenheimer approximation (BOA) is the foundation for understanding the quantum nature of molecular systems and leads to the development of important concepts such as electronic states and molecular orbits. In a molecule, non-adiabatic interactions between electronic states are ubiquitous. However, because of the complicated nature of non-adiabatic couplings, molecular systems are often treated without considering non-adiabatic couplings and the effect of excited states.
However, in the presence of conical interactions in molecular systems, such approximations could break down. Half a century ago, scientists found that by introducing a geometric phase, one could properly treat these systems quantum mechanically. Introducing a GP effect, however, could have a profound effect on the quantum systems. For example, one of the quantum Hall effects results from an electronic geometric phase effect. Therefore, the effect of the geometric phase is a fundamental question in both physics and chemistry.
The research work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences and the Ministry of Science and Technology.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

