
A team of Prof. Eugene Gregoryanz, Dr. LIU Xiaodi and Prof. CHEN Xiaojia etc. from Institute of Solid State Physics, Hefei Institutes of Physical Science, presented the first high-pressure low-temperature experimental study of hydrogen which was completely conducted within China.
According to the study, a novel phase, phase II', unique to deuterium, was found and an updated phase diagram of hydrogen (deuterium) at high pressures and low temperatures was demonstrated. Their work was published in Physical Review Letter.
Hydrogen, the first element in the periodic table as well as the most abundant element in the Universe, is very important for the research in Physics, Chemistry, geophysics, geophysiography, etc.
The hydrogen research at high pressure have been an alluring topic for several decades, due to its unusual and fantastic properties and applications which was predicted theoretically, such as room temperature Tc superconductor, super-fluid group state, rocket fuel, etc.
Despite numerous theoretical and experimental studies on hydrogen at very high pressures recently, there is less attention paid to the hydrogen at moderate pressure (0-200 GPa) and low temperature (4-300 K). For several decades, three phases (Phase I, II, III) were believed to existed in this area.
Based on this, the studies at moderate pressure and low temperature are very important to understand the evolution of the system during the transformation from weak intermolecular interaction phase (phase I,II, III) to strong intermolecular phase(phase IV,V).
Prof. Eugene Gregoryanz and Dr. LIU Xiaodi focus on the study of hydrogen at high pressures (0-220 GPa) and low temperatures (4-300 K).
By using diamond anvil cell static compression technique, they performed in situ high-pressure low temperature Raman measurement for both isotopes which demonstrate the presence of a novel phase, phase II', unique to deuterium and distinct from the known phase II.
However, Phase II' of D2 is not observed in hydrogen, making it the only phase that does not exist in both isotopes and occupies a significant part of P-T space from ~25 to 110 GPa and below 125 K.
The phase diagram of hydrogen and deuterium were also revisited.
For H2, the data shows that below 30 K the transition to phase II happens at as low as 73 GPa. The transformation from phase II to III commences at around ~155 GPa and is completed by 170 GPa with the average pressure of ~160 GPa being slightly higher than previously thought. The updated phase diagrams of H2 and D2 demonstrate the difference between the isotopes at low temperatures and moderate pressures, providing new information on the phase diagrams of both elements.
This work is one of the breakthrough of hydrogen research at medium pressures and low temperatures for the nearly 3 decades, which will throw profound impact on understanding the basic laws of Physics, the phase diagram of hydrogen (deuterium), the quantum effect in phase II,etc.
This work was supported by Thousand Talents program, the National Natural Science Foundation of China, Natural Science Foundation of Anhui Province, China.
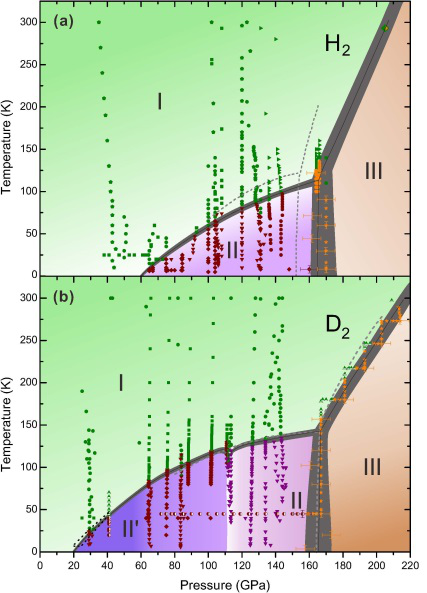
Proposed phase diagrams of hydrogen (a) and deuterium (b) in a low-temperature medium-pressure range. (Image by LIU Xiaodi)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

