Graphene is one of the lightest, strongest, and highest-conductivity materials in existence. Since it was
introduced to the world in 2004, many scientists have focused on understanding and harnessing the incredible potential of this two-dimensional form of carbon—but the discovery of graphene also kicked off a search for similar forms of other elements, in hopes that they might have unique and valuable properties as well.
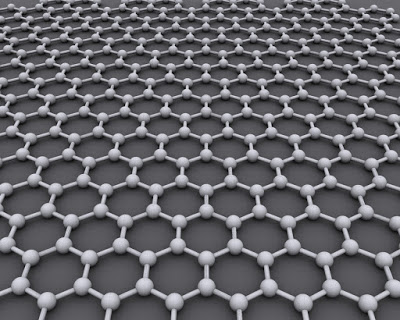
Graphene is formed by an atomic-scale hexagonal pattern of carbon atoms.
Image Credit: Graphene by AlexanderAlUS - Own work, CC BY-SA 3.0.
Last week in the American Physical Society’s newest journal,
Physical Review Materials, a team of Chinese researchers
shared their latest work on borophene, a recently discovered graphene-like form of the element boron. For the first time, the team created long, thin ribbons of borophene called nanoribbons. These nanoribbons are expected to have different electronic properties than sheets of borophene and represent a milestone on the path to electronic devices based on boron.
Boron has chemical properties similar to carbon, and the two elements sit right next to each other on the periodic table. Like carbon, boron is essential for plant and animal life (although in much smaller amounts). It’s also likely responsible for any green fireworks you’ve seen recently, and it’s is an essential ingredient in many household cleaners and solutions.
Borophene was grown just a few years ago for the first time and has displayed remarkable electronic properties. This new research on borophene nanoribbons was carried out by one of the teams that first synthesized borophene. During the initial investigation, the team noticed that the one-atom-thick sheets of boron aligned in a particular way with the silver surface on which they were grown. In other words, the atomic structure of the silver surface influenced how the borophene grew.
This led the team to wonder how the borophene would change if it was grown on a surface with a different atomic structure; in particular, on a less symmetric surface. So, the team grew borophene again, but this time on a silver surface with fewer symmetries. The result was several parallel nanoribbons that were 10.3 nanometers wide (one ten-thousandth the width of a human hair) and up to hundreds of nanometers long.
Nanoribbons aren’t just cool, they have enormous potential for electronic devices—research on ones made of graphene shows that nanoribbons with different geometries have different electronic properties. In other words, you can create nanoribbons with the electronic properties you want by controlling their geometry. The stumbling block for graphene nanoribbons is that, at least so far, they have been difficult to manufacture. Boron nanoribbons don’t appear to present this same challenge.
Although the nanoribbons were easy to grow by comparison, they were difficult to understand. “We spent about one month on the experiments, but about one year on the theoretical explanations on the atomic structures of borophene nanoribbons,” says researcher Lan Chen from the Institute of Physics, Chinese Academy of Sciences.
The challenge was that high-resolution images of the nanoribbons revealed four different atomic structures, or repeating patterns. Some ribbons were composed of just one pattern while others had multiple. This confused the team—until they went out for barbecue one night.
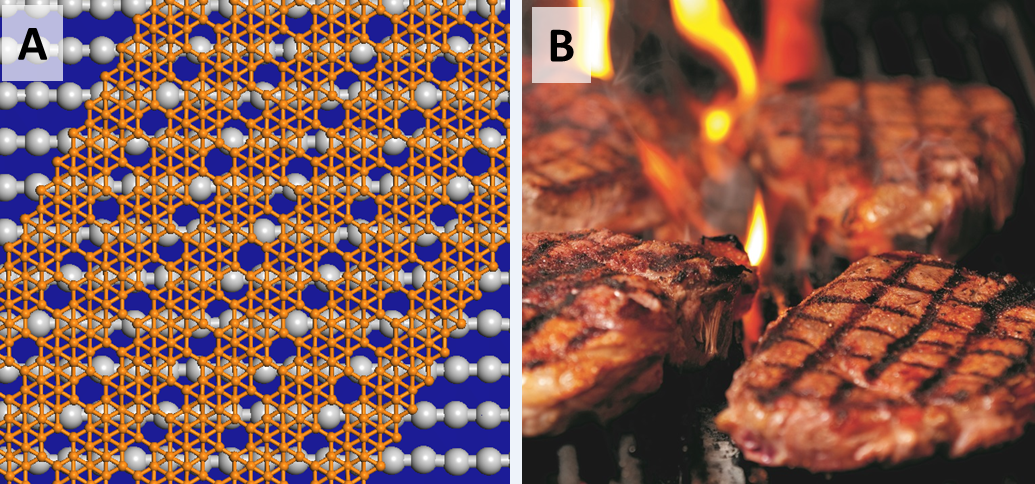
(A) Atomic model of striped borophene nanoribbon lying on a silver surface, similar to (B) grill marks on steaks. Image Credit: Courtesy of Lan Chen.
"We noticed that our borophene nanoribbon structures are very similar to roast meats on barbecue grills," said Li and Chen. The silver surface consists of parallel silver chains, while the borophene nanoribbons consist of boron chains. As with grill marks, different widths and relative positions of boron chains with respect to the silver chains result in different patterns. “Based on this principle, we figured out the atomic structures of borophene nanoribbons very quickly," said Hui Li from Beijing University of Chemical Technology.
Borophene nanoribbons are predicted to have intriguing electronic properties, like graphene, but they haven’t been studied yet. The team is current examining the electronic structure of the nanoribbons, says Chen, and will then move on to studying their electronic properties. This should provide insight on whether we might see boron-based devices someday. Stay tuned! (physics central)








