
Structure of the full-length human glucagon receptor ignites new excitement in GPCR research
A team of scientists from Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences has determined the high-resolution atomic structure of a full-length class B G protein-coupled receptor (GPCR) that plays a key role in glucose homeostasis. This structure revealed, for the first time, the structural framework of different domains of a class B GPCR at high resolution and unexpectedly disclosed many exciting molecular features, greatly deepening the understanding of signaling mechanisms of class B GPCRs.
In this study, scientists from SIMM in collaboration with scientists from ShanghaiTech University, Zhengzhou University, Fudan University and foreign institutions provided a detailed molecular map of the full-length human glucagon receptor (GCGR) in complex with a negative allosteric modulator (NNC0640) and the antigen-binding fragment of an inhibitory antibody (mAb1). This study was published online in Nature with a companion paper led by colleagues at the iHuman Institute, ShanghaiTech University describing the glucagon-like peptide-1 receptor (GLP-1R).
Class B GPCRs are essential to numerous physiological processes and serve as important drug targets for many human diseases. The GCGR structure was found by scientists to provide a clear picture of a full-length class B GPCR at high resolution, and help people understand how different domains cooperate in modulating the receptor function at the molecular level.
Class B GPCR receptors consist of an extracellular domain (ECD) and a transmembrane domain (TMD), both of which are required to interact with their cognate peptide ligands and to regulate downstream signal transduction. Due to difficulties in high-quality protein preparation, structures of full-length class B GPCRs remained elusive, thus limiting a comprehensive understanding of molecular mechanisms of receptor action.
This study gave some valuable insights into the structure of GCGR. The most exciting finding is that the linker region connecting the ECD and TMD of the receptor, termed the “stalk”, works together with an extracellular loop of the TMD to regulate peptide binding through conformational changes, serving like a modulator in receptor activation. "Although the stalk region only contains 12 amino acids, it acts as a ‘switch’ to turn on or turn off the receptor,” said Dr. WU Beili from SIMM. “It is amazing to observe how a GPCR regulates its function in such a precise and efficient way.”
Based on the full-length GCGR structure, researchers performed a series of functional studies using hydrogen-deuterium exchange, disulfide cross-linking, competitive ligand binding and cell signaling assays as well as molecular dynamics simulations. The results supported the GCGR structure and confirmed the interactions between different domains in modulating its functionality via conformational alterations.
"The full-length GCGR structure not only expands the knowledge about GPCR signaling mechanisms, but also offers new opportunities in drug discovery targeting class B GPCRs,” said Dr. WANG Mingwei, Director of the National Center for Drug Screening. “With the information gained from this structure, we are in a better position to devise new therapeutic strategies involving both GCGR and glucagon-like peptide-1 receptor for obesity and type 2 diabetes.”
The study was funded by the National Basic Research Programs, the National Health and Family Planning Commission, the National Natural Science Foundation, Chinese Academy of Sciences, Shanghai Science and Technology Development Fund, GPCR Consortium and National Institutes of Health (U.S.A.).
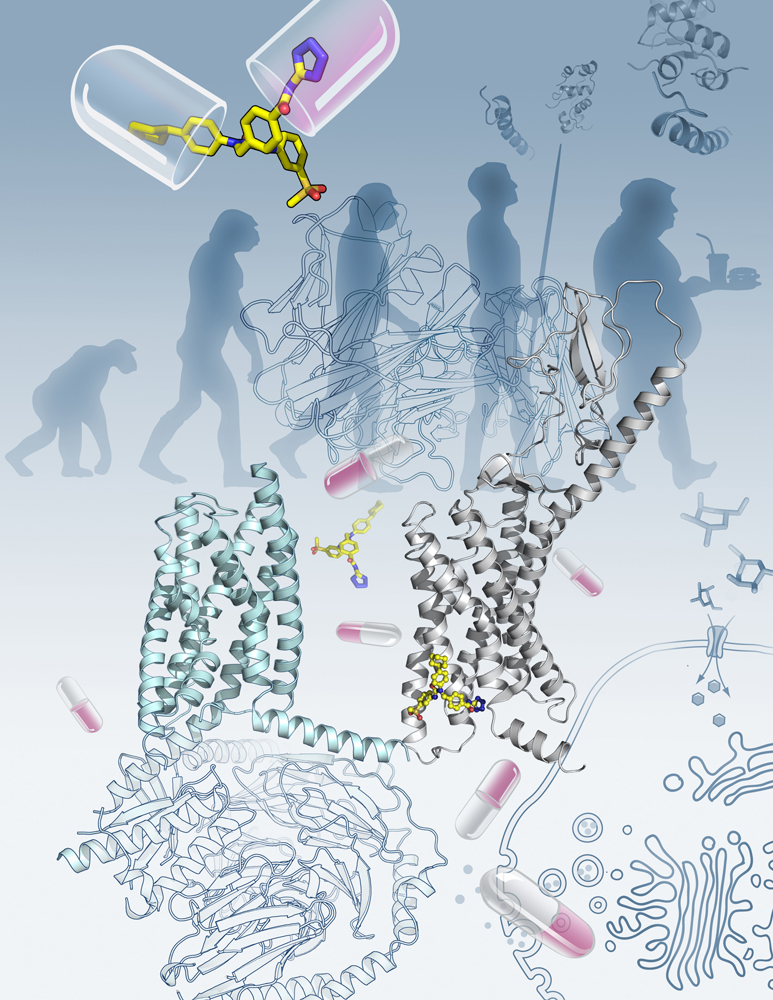
Figure: The crystal structure of the full-length human glucagon receptor (GCGR) plays a key role in glucose homeostasis and serves as an important drug target for type 2 diabetes. (Image by Yekaterina Kadyshevskaya of the Bridge Institute at the University of Southern California)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

