
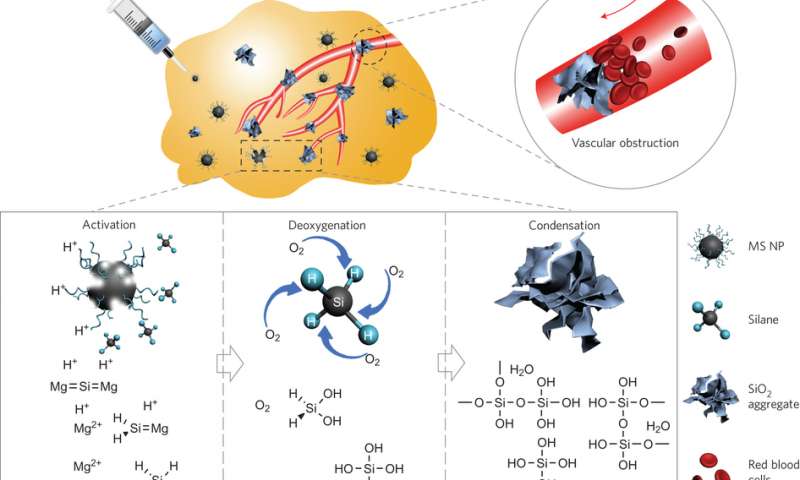
Schematic diagram of MS NPs serving as an intratumoral DOA for specific cancer-starving therapy. Activated by the acidic tumour microenvironment, the MS NPs produce reactive silane to give rise to an efficient deoxygenation effect and produce in situ SiO2 blockers in tumour blood capillaries, which subsequently prevent undesired reoxygenation. The deoxygenated tumour with no further oxygen supply will suffocate in the absence of the necessary energy metabolism. MS NP here is the PVP-modified Mg2Si nanoparticles.
One target therapy in cancer research is to suffocate the tumor. Cells need oxygen to survive so researchers have focused on methods for cutting off the blood supply to the tumor. Very little research has involved the direct removal of oxygen within the tumor.
To this end, a group of researchers from the Shanghai Institute of Ceramics, Chinese Academy of Sciences and the East China Normal University have developed a deoxygenation agent using polyvinyl pyrrolidone modified Mg2Si nanoparticles. This agent is pH sensitive, efficiently consumes oxygen, and one of the products of oxygen consumption also forms aggregates that could potentially block blood vessels. Preliminary mouse studies show tumor hypoxia and good biocompatibility. Their work appears in Nature Nanotechnology.
There are several important qualities for a good tumor-starving agent. For one, the agent must be biocompatible which negates the use of heavy metals for oxygen absorption. Additionally, the agent must be efficient at deoxygenation and serve as a long-term oxygen scavenger including preventing re-oxygenation of deoxygenated tumors through undamaged blood vessels. And, as always, any cancer treatment needs to target tumors without damaging healthy tissue, and the agent should be easily injectable by syringe.
In the current research, Zhang et al. developed polyvinyl pyrrolidine (PVP)-modified Mg2Si nanoparticles that have several of the qualities for a good tumor-starving agent. Importantly, the main components, magnesium, silicon dioxide, and water are biocompatible. Additionally, the reaction mechanism forms a highly reactive O2 scavenger, SiH4, which serves to make these nanoparticles highly efficient at scavenging oxygen.
In order to make injectable nanoparticles, Zhang et al. developed a self-propagating high-temperature synthesis in an oxygen-argon atmosphere. This allows the nanoparticles to remain dispersed in the liquid, rather than form clusters, so that they are injectable into tissue. This synthesis takes advantage of the formation of MgO by-product that halts the continual formation of Mg2Si aggregates.
Part of the reaction mechanism involves the formation of Si4-, which is highly sensitive to acid. This is important because the tumor environment tends to be acidic compared to normal tissue (pH~6.4), and pH sensitivity may help with tissue specificity. To investigate the pH sensitivity of their deoxygenation agent, Zhang et al. placed their nanoparticles in a dialysis bag, which were then immersed in buffer solutions of varied pH values within closed tubes. In acidic conditions, the nanoparticles irreversibly decreased the level of oxygen, but were unreactive in neutral pH. Furthermore, SiO2 aggregates formed in situ that served to block a simulated capillary.
Additional studies demonstrated that the MgSi2 nanoparticles demonstrated very little cytotoxicity until they encountered the acidic environment of the cancer cell. Using MCF-7 human breast adenocarcinoma cells, Zhang et al. observed that the combination of acid and nanoparticles led to cell efficient hypoxia. Furthermore, cell proliferation decreased, which is likely due to mitochondrial damage from deoxygenation.
In vivo studies involving bilateral 4T1 xenotumor-bearing mice demonstrated that Mg2Si nanoparticles served as efficient deoxygenation agents. Each mouse was injected with the nanoparticle deoxygenation agent in the right tumor and with saline solution as a control in the left tumor. Measurements of blood oxygen saturation levels after ten minutes showed little change in the control tumor and a drastic reduction in oxygen in the test tumor. Oxygen reduction continued for three hours in the tested tumor until tests on both the hemoglobin-bound oxygen and blood oxygen showed complete depletion within the tumor. Notably, PET/CT images show that hypoxia occurred within the tumor and not in the surrounding tissues.
Additional observations from the in vivo study showed that the tumors that received Mg2Si nanoparticles demonstrated a slower growth rate compared to controls and after twenty-four hours, although cell proliferation was not slowed down as significantly as in the in vitro studies. These cells showed evidence of fibrosis, necrosis, and apoptosis. Additionally, magnesium was quickly eliminated from the body while silicon was eventually eliminated.
Overall, this research provides a compelling proof-of-concept for the use of PVP-modified Mg2Si nanoparticles as potential candidates for use as a tumor-targeted deoxygenating agent. The authors point out that future research would involve exploring surface modifications of the nanoparticles to tailor the length of time the nanoparticles can travel through the blood stream. (Phys.org)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

