
The increasing environmental pollution by widespread heavy metals is of great concern due to their extreme toxicity, inability to be biodegradable, and tendency to be accumulated in the body via the food chain, which pose a long-term adverse threat to human health and natural ecosystems. The adsorption technique, as one of the most potential methods to remove metal ions, has received considerable interest due to its wide adaptability, easy handling, comparably low cost, and the availability of different adsorbents. Accordingly, various adsorbents such as activated carbon, zeolites, minerals (clays), molecular sieves and inorganic nanomaterials have been extensively studied to remove heavy metal pollutants from aqueous solutions. However, these adsorbents usually suffer from low removal capacities, slow capture kinetics, difficult separation, secondary environmental pollution from finer-sized materials and unsatisfactory recycling ability, which greatly limit their practical applications.
To tackle the issues mentioned above, a research group in Centre of Environmental and Energy Nanomaterials (CEEN), Institute of Solid State Physics, Hefei Institutes of Physical Science, made progress in fabrication of novel three-dimensional (3D) graphene/δ-MnO2 aerogels via self-assembly and reduction of graphene oxide, followed by in situ solution-phase deposition of ultrathin δ-MnO2 nanosheets to remove heavy metal ions in wastewater purification. The related work was published in Journal of Materials Chemistry A entitled 3D graphene/δ-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions.
The research group fabricated graphene/δ-MnO2 architectures which show an interconnected 3D network microstructure in which a large number of ultrathin birnessite MnO2 nanosheets are homogenously deposited on the graphene framework.
Due to their unique structural characteristics, the resulting 3D aerogels exhibit a fast adsorption kinetic rate and superior adsorption capacity toward heavy metal ions. The saturated adsorption capacities of graphene/δ-MnO2 aerogels are as large as 643.62 mg g-1 for Pb2+, 250.31 mg g-1 for Cd2+and 228.46 mg g-1for Cu2+calculated by the Langmuir isotherm model, exceeding largely the corresponding pristine 3D graphene and δ-MnO2 nanosheets.
It is noted that the heavy metal ions could not only adsorb on the surface of graphene/δ-MnO2, but also intercalate into the interlayer gaps of birnessite MnO2, which are the synergistic effect of the static electrical attraction, surface complexation and ion exchange between heavy metal ions and pre-intercalated K+. These results were supported by the expansion of the basic crystal structure of layered MnO2 after adsorption.
Furthermore, it is interesting that the regenerated aerogels after the initial HCl and subsequent KOH treatment still maintain their original shape and can be repeatedly used for more than eight cycles without obvious degradation of performance, which achieved the sustainability of the absorbents.
More importantly, the hybrid aerogels can be easily separated and do not generate secondary contaminants. Thanks to the high removal efficiency, fast adsorption kinetics, excellent regeneration and reusability and ease of separation operation, these hybrid aerogels are considered as ideal candidates for heavy metal ion decontamination in practical application.
This study was sponsored by the National Basic Research Program of China, National Natural Science Foundation of China and CAS/SAFEA International Partnership Program for Creative Research Teams of Chinese Academy of Sciences, China.
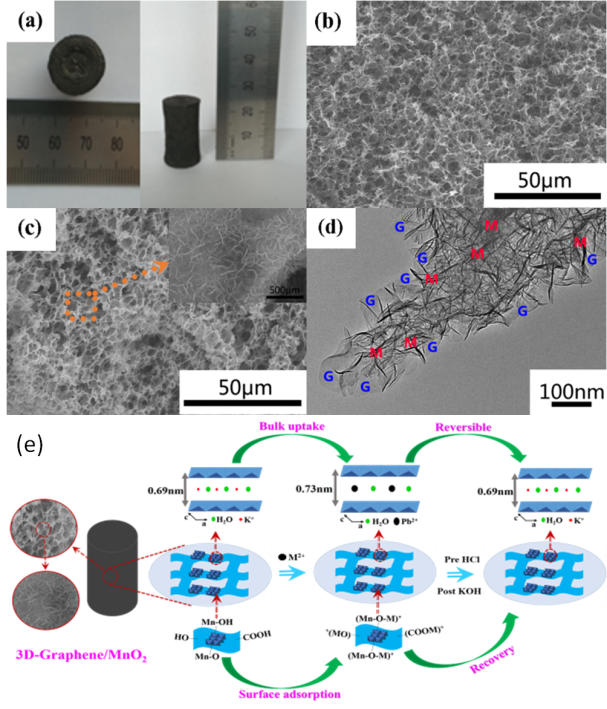
(a) Photographs of 3D graphene aerogels; (b) SEM image of 3D graphene aerogels; (c) SEM images of graphene/MnO2; (d) TEM image of the graphene/MnO2 (graphene sheets are marked in blue G and MnO2 sheets are marked in red M); (e) Schematic illustration of the preparation process of 3D graphene/MnO2 aerogels (Image by ZHOU Hongjian)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

