
Translationally-controlled tumor protein (TCTP) is conserved in eukaryotes and is an abundant protein involved in cellular growth, differentiation, apoptosis, and in the progress of carcinogenesis and inflammation. In 2007, prof. FENG Yingang at the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of Chinese Academy of Sciences, reported the solution structure and calcium-binding site of human TCTP. After that, he has continued to systematically study the interactions between TCTP and other proteins.
Prof. Feng and prof. Sarah Perrett at the Institute of Biophysics (IBP) collaborated in the study of the interaction between TCTP and eukaryotic elongation factor 1B (eEF1B) using nuclear magnetic resonance and isothermal titration calorimetry techniques. They identified the key binding regions and amino acid residues involved in the interaction of the proteins, and calculated a model of their complex based on the experimental data. The work revealed that the interaction between TCTP and eEF1B is one of primary biological functions of TCTP. This study has been published on J. Biol. Chem.
The results indicate that a newly identified central acidic region (CAR) domain of eEF1Bδ is responsible for TCTP binding, and this CAR domain binds to TCTP through hydrophobic and electrostatic interactions. Further sequence analysis indicates that the CAR domain is conserved in all guanine nucleotide exchange factors of the eEF1B complex in eukaryotes.
The interaction between TCTP and eEF1B was further confirmed using TCTP and eEF1Bα homologues from two lower eukaryotic species (fission yeast and unicellular photosynthetic microalga). The interaction of TCTP and eEF1B guanine nucleotide exchange factors is conserved in eukaryotes which implies that TCTP is involved in the protein translation process and that this function represents the primary function of TCTP.
Many interactions have been reported for TCTP without structural information. This study provides a paradigm for further studies of the structural mechanism of these interactions.
The research project was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and the Beijing Natural Science Foundation.
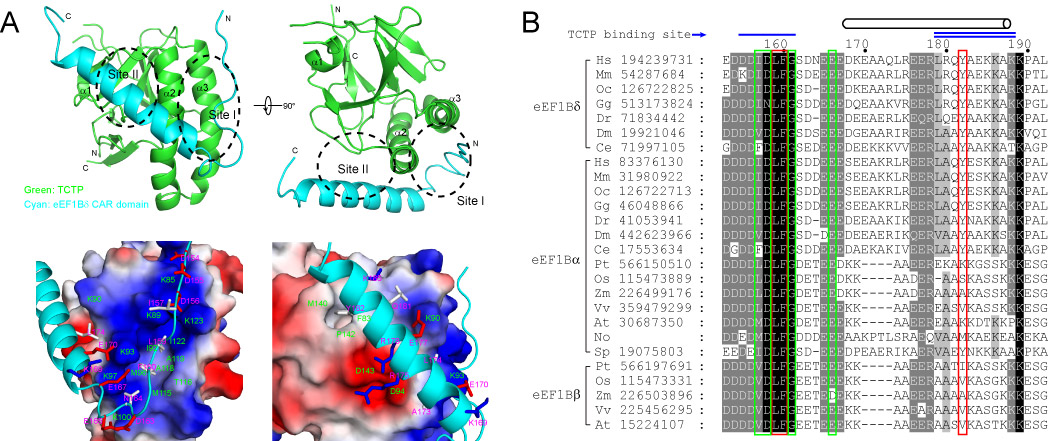
Figure: (A) Structure model of the complex of TCTP and eEF1Bδ CAR domain. (B) Conserved TCTP-binding sites on CAR domains of various eEF1B guanine exchange factors. (Image by QIBEBT and IBP)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

